Attempted
Correct
UnAttempted
Wrong
Listen to the following audio and retell lecture in your own words.
NOTE:- TRANSCRIPT (Only for reference, it will not be given in actual PTE Academic Test)
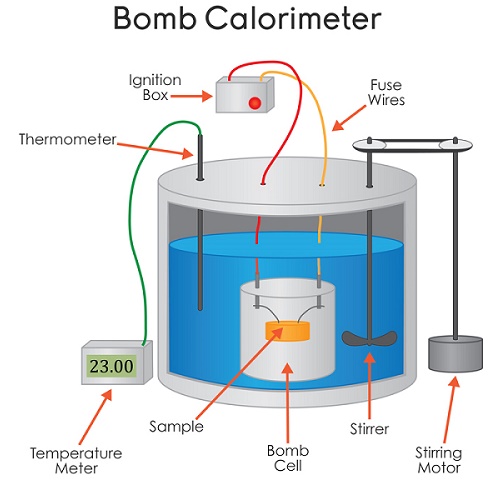
This is a bomb calorimeter this is the actual piece of equipment that research reviews to calculate the energy content of either bio-diesel or maybe even the potato chips that you had for lunch today.
When they calculate the amount of energy they’re going to calculate it in heat units which would either be joules or calories. I want you to look inside the bomb calorimeter inside here you can see that there’s a silver bucket, water goes all in here and this is actually the bomb is the smaller silver cylinder what you do is put your fuel sample in there then. These two electrodes are connected to the bomb these provide the spark that will ignite your sample when your sample burns or combust that gives off energy so how is the energy collected or how did how does a scientist figure out how much energy is being given off. Well it’s a closed system there’s a lid here that goes on top of this calorimeter and what’s in here in the lid is a stir the stir is going to stir
the water that’s in this big pool here so that the heat given off from the sample is going to warm the water in a uniform way this is the temperature probe this goes down in the water off so and measures the change in temperature because as the sample is burned it will give off heat and the temperature of the water will increase so the lid goes on the sample is prepared the last thing that you need to
make a combustion reaction happen is oxygen and at some point during the process some oxygen is added by a tank that’s connected to the calorimeter here so we are going to burn a sample of the bio-diesel that you’ve prepared and get some feedback on the energy content of it you’ll be able to use this to compare it to petroleum-based fuels like octane